Abstract
Background:DNMT3A mutation can be seen in premalignant hematopoietic stem cells and is mainly associated with clonal hematopoietic of indeterminant potential (CHIP), whereby prevalence increases with age. The mutation has been predominantly observed in AML with NPM1 mutation and co-occurrence with FLT3-ITD. The prognostic significance of cooperating mutations with DNM3TA mutation remains controversial. The objective of our study was to identify characteristics and factors that affect the outcome of DNMT3A cooperating mutations in de novo AML across the age spectrum utilizing a large cohort of patients enrolled on several pediatric and adult trials.
Methods: The total cohort (N=3142) included patients from <1 month to 88 years old. Pediatric and young adults were treated on multiple AML trials including CCG-2961 (n=23), AAML03P1 (n=54), AAML0531 (n=733), AAML1031 (n=1062), (total peds, n=1872) and adult patients on SWOG (n=359), BEAT AML (n=333), TCGA (n=180), and ECOG-ACRIN (n=398) (total adults, n=1270). Mutational profiling was performed by whole-genome, transcriptome, and targeted sequencing and prevalence of gene mutations were analyzed based on age groups. Prevalence of DNMT3A mutation, co- occurring mutations as well as the outcomes were analyzed based on cooperating mutations.
Results: While the prevalence of DNMT3A mutation among the entire cohort was 8.4% (N=264), only 2 cases were identified in pediatrics and young adult patients (0-18 years old) and prevalence of DNMT3A mutation amongst the adult cohort was 20.67%. The mutational prevalence was correlated with increased age (p value <0.05). Missense mutations at residue 882 were most prevalent (n=165) while other missense, nonsense and frameshift mutations were identified throughout the gene (n=99). A comparison of canonical R882 vs other mutations found no difference in overall survival (p=0.7924).
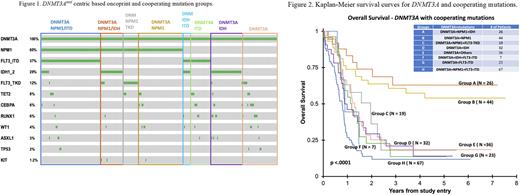
DNMT3A centric oncoprint analysis demonstrated that DNMT3A mutation commonly co-occurred with NPM1 mutation (60%), followed by FLT3-ITD (37%), IDH1/2 (29%) and FLT3-TKD mutations (12%) (Figure 1). Based on the DNMT3A and cooperating mutations, the patients were divided into 8 groups and the overall survival was analyzed (groups A-H). Although majority of DNMT3A positive patients had adverse outcome, those with DNMT3Amut/NPM1mut and DNMT3Amut/NPM1mut/IDHmut groups without other commonly associated mutations did well (p value <0.0001, 54.2% and 62.9% 5 year OS estimates, respectively). Specifically, presence of other mutations including FLT3-TKD with DNMT3Amut/NPM1mut results in a lower OS (group C, 18.1% 5-year OS estimate). DNMT3Amut co-occurring with any other gene mutations (other than NPM1) including DNMT3Amut/FLT3 ITDmut have the lowest OS with 5 year OS estimates of 13.7%, 13.9%, and 18.4%, groups F-H, respectively (Figure 2). The most dismal outcomes were observed in the DNMT3Amut/NPM1mut/FLT3 ITDmut patients with 5 year OS estimates of 11.8%.
Conclusion: This large cohort study confirms that prevalence of DNMT3A gene mutation increases with age and is a rare event in pediatrics and young adult patients. While DNMT3A mutation doesn't have an independent prognostic factor by itself, we demonstrated that co-occurring NPM1 mutation has a favorable outcome even in the presence of IDH1/2 mutation. However, co-occurring DNMT3Amut with IDH1-2 mutation without NPM1 has an inferior OS, highlighting the prognostic impact of NPM1 mutation. Presence of FLT3 ITDmut has an unfavorable OS, regardless of NPM1 and/or IDH1/2 mutations.
Disclosures
Othus:Daiichi Sankyo: Consultancy; Glycomimetics: Consultancy; Biosight: Consultancy; Merck: Consultancy; Celgene: Consultancy. Appelbaum:Jasper Biotherapy: Membership on an entity's Board of Directors or advisory committees. Erba:Incyte: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; MacroGenics: Consultancy, Research Funding; Novartis: Consultancy, Research Funding, Speakers Bureau; Covance (Abbvie): Consultancy, Other: Independent Review Committee, Research Funding; Janssen Oncology: Consultancy; Trillium Therapeutics: Consultancy; Takeda: Consultancy; Amgen: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Glycomimetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Celgene: Consultancy, Other, Speakers Bureau; Astellas Pharma: Consultancy; Agios: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kura Oncology: Consultancy; Forma Therapeutics: Research Funding; Gilead/Forty Seven: Research Funding; PTC therapeutics: Research Funding; ALX Oncology: Research Funding; Pfizer: Consultancy. Tallman:UpToDate: Patents & Royalties; Syros Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Kura: Membership on an entity's Board of Directors or advisory committees; Innate Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Ipsen Biopharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Oncolyze: Membership on an entity's Board of Directors or advisory committees; KAHR-Adv Bd: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Rafael Pharmaceuticals: Research Funding; Glycomimetics: Research Funding; Biosight: Membership on an entity's Board of Directors or advisory committees, Research Funding; Orsenix: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Atallah:Blueprint: Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau; Takeda: Research Funding; Novartis: Consultancy, Research Funding; BMS: Consultancy, Speakers Bureau. Luger:Onconova, Celgene, Biosight, Hoffman LaRoche, and Kura: Research Funding; Syros, Agios, Daiichi Sankyo, Jazz Pharmaceuticals, Brystol Myers Squibb, Acceleron, Astellas, and Pfizer: Honoraria. Abdel-Wahab:H3B Biomedicine, LOXO Oncology, and Nurix Therapeutics: Research Funding; H3B Biomedicine, Foundation Medicine Inc, Merck, Prelude Therapeutics, and Janssen: Consultancy; Envisagenics Inc., AIChemy, Harmonic Discovery Inc., and Pfizer Boulder: Membership on an entity's Board of Directors or advisory committees. Levine:Ajax, Abbvie, Constellation, Zenalis, Celgene, Roche, and Prelude: Other: research support; Qiagen: Other: supervisory board member; Syndax, Incyte, Janssen, Astellas, Morphosys and Novartis: Consultancy; Astra Zeneca and Kura: Other: honoraria for invited lectures ; Imago, Mission Bio, Bakx, Zentalis, Ajax, Auron, Prelude, C4 Therapeutics and Isoplexis: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Gilead and Novartis: Other: Grant reviews.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal